For couples wondering "why do I keep having all boys" or "is there a genetic test to predict if I'll have all girls," a groundbreaking Harvard study now provides answers. The long-held view that the sex of human offspring is determined solely by the paternal contribution of an X or Y chromosome has stood as a foundational tenet of reproductive biology. However, epidemiological observations of families producing exclusively male or female children suggest that sex determination at birth may be more complex than a simple 50:50 chromosomal lottery. While stochastic variation can account for some clustering, the persistent overrepresentation of unisexual sibships across populations hints at underlying biological biases.
Recent advances in genomics have enabled systematic interrogation of these biases at the population level, potentially leading to genetic tests that can predict whether someone is predisposed to have children of predominantly one gender. In this context, the present study leverages data from 58,007 U.S. women and 146,064 singleton live births to perform the first genome-wide association study (GWAS) focused specifically on maternal genetic determinants of offspring sex clustering. The analysis reveals that sex distributions within families deviate significantly from binomial expectations, instead fitting a beta-binomial model—implying that the probability of male or female birth is not constant across the population, but varies between individuals.
The GWAS identifies several loci with genome-wide significant associations to unisexual sibships (families with all boys or all girls), most notably variants near NSUN6, TSHZ1, and CYP2U1. These genetic markers could form the basis of future DNA tests to determine if women have a genetic predisposition to have children of only one sex. These findings provide the first direct genetic evidence that maternal nuclear DNA can bias offspring sex ratios, independent of paternal contribution. Such loci may influence early developmental processes, from gamete–zygote interactions to implantation success, in a sex-specific manner.

The identification of these genetic variants opens new avenues for understanding reproductive biology. While the mechanisms remain to be fully elucidated, the convergence of statistical evidence and biological plausibility suggests that maternal genetics plays a more active role in sex determination than previously recognized. These findings challenge the traditional view that offspring sex is determined solely by whether a sperm carries an X or Y chromosome.
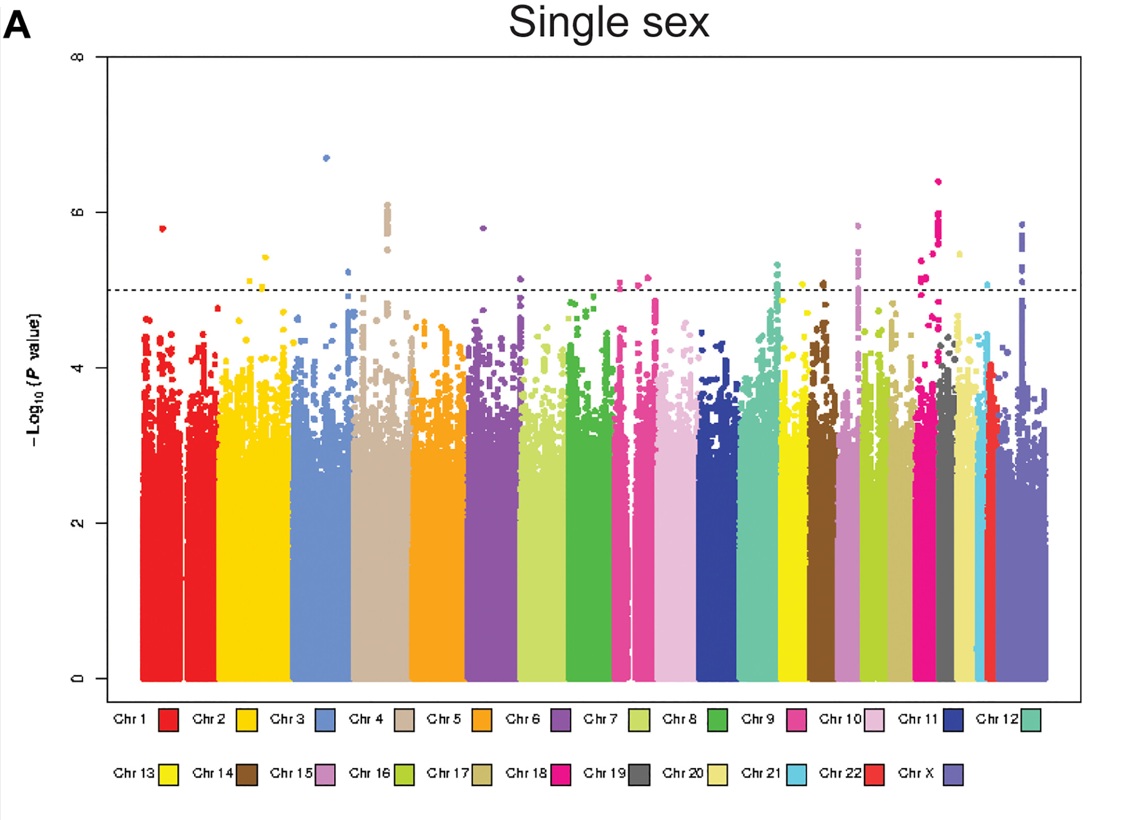
This chart shows a genome-wide association study (GWAS), which is essentially a scan across the entire human genome to see where genetic differences in mothers might be linked to having children of only one sex. Each colored block represents one of the 22 human chromosomes (plus X), and each dot is a genetic variant tested in the study. The higher a dot is on the graph, the stronger the statistical link between that variant and the pattern of having all sons or all daughters.
The dashed line marks the "genome-wide significance" threshold — dots above this line are considered strong evidence of a real connection rather than random chance. A few peaks rise above the line, meaning these regions of the genome are especially interesting for scientists to investigate. These peaks may contain genes that subtly affect early pregnancy in ways that favor one sex over the other.
This figure illustrates the distribution of association signals across the genome, with clear peaks surpassing the genome-wide significance threshold, underscoring the robustness of the associations detected. Together, these findings open a new frontier in the functional genetics of sex determination, reframing maternal biology as an active, probabilistic participant in shaping the sex of offspring.
Results
Sex Ratio Clustering and Departure from Binomial Expectations
Across 146,064 singleton live births from 58,007 U.S. women, the distribution of offspring sex within families consistently diverged from the null expectation of a simple binomial process (P < 0.001 for all sibship sizes ≥ 2, χ² goodness-of-fit). In a purely random model, the probability of a male birth (~51.9% in this cohort) should remain constant and independent of previous births. Instead, the observed patterns aligned more closely with a beta-binomial distribution, a statistical signal of overdispersion—indicating that some families possess an intrinsic bias toward sons or daughters beyond what chance alone would dictate.
This bias became increasingly evident in larger families. While two-child households skewed toward mixed-sex compositions, sibships of three or more were disproportionately unisexual, a trend that persisted even after excluding the final birth from each family to minimize the effect of "sex-based stopping" behavior (the so-called coupon collection phenomenon). The persistence of this deviation under these controls supports a biological basis for the clustering, rather than one solely driven by reproductive decision-making.
From Statistical Signal to Genetic Association
The observed overdispersion prompted an investigation into possible genetic underpinnings using genome-wide association analysis. Restricting the analysis to 7,530 genotyped participants from the Nurses' Health Study II (NHSII), the GWAS revealed multiple loci surpassing the conventional genome-wide significance threshold (P < 5 × 10⁻⁸), with association peaks on chromosomes 4, 10, and 18 (Figure A).
The most significant association for female-only sibships occurred at NSUN6 (Chr10, rs58090855; P = 2.7 × 10⁻⁸). NSUN6 encodes a tRNA methyltransferase essential for maintaining RNA stability and translational fidelity—functions that could plausibly intersect with sex-specific differences in early embryonic development or implantation efficiency. For male-only sibships, the strongest signal arose at TSHZ1 (Chr18, rs1506275; P = 4.6 × 10⁻⁸), a zinc-finger transcription factor with a critical role in neural crest development and possibly in pathways influencing placental formation and early fetal survival in a sex-biased manner. A cluster of variants in CYP2U1 (Chr4), a gene central to fatty acid metabolism and mitochondrial function, also showed suggestive associations, raising the possibility that maternal metabolic state could subtly shape the uterine environment in ways that favor one sex over the other.
Importantly, none of these loci overlap with established genetic determinants of reproductive timing—such as age at menarche or menopause—suggesting that they represent novel biological pathways influencing sex ratio bias rather than secondary effects of known reproductive traits.
Magnitude of Effect and Demographic Impact
When modeled within the beta-binomial framework, the presence of sex clustering translated into measurable shifts in conditional birth probabilities. In families with three sons, the probability of a fourth son rose to approximately 61%; in families with three daughters, the probability of a fourth daughter was roughly 58%. Though modest in absolute terms, these deviations become demographically significant when considered at population scale, particularly over multiple generations and in cultural contexts where existing preferences for one sex could amplify the effect.
Summary of Integration
Taken together, the statistical evidence of overdispersion and the identification of genome-wide significant maternal loci provide a coherent narrative: sex at birth is not strictly a chromosomal coin toss, but a probabilistic outcome shaped in part by maternal genetic architecture. The data reveal that while the effect size at the individual level is small, the pattern is robust, reproducible under multiple sensitivity analyses, and biologically anchored in genes with plausible roles in early development and implantation.
What This Means for Genetic Testing and Family Planning
These findings challenge the prevailing model in which paternal gametes alone determine the sex of offspring, revealing that maternal nuclear DNA may subtly influence the outcome. For parents who have wondered "can a genetic test tell me if I'll have all boys or all girls," this research suggests such tests may soon be possible. The genome-wide significant associations implicate genes with functions that plausibly intersect with early developmental processes known to exhibit sex-specific vulnerability. For example, NSUN6, a tRNA methyltransferase, maintains RNA stability and translational fidelity—processes that could affect the viability of embryos in a sex-dependent manner, possibly through differences in metabolic demands or epigenetic programming in early cleavage stages. TSHZ1, a zinc-finger transcription factor crucial to neural crest development, may contribute to sex-biased placental development or signaling at the maternal–fetal interface, influencing implantation success or early survival probabilities. Likewise, CYP2U1, which regulates fatty acid metabolism and mitochondrial activity, could alter the intrauterine biochemical environment in ways that differentially affect male and female embryos, consistent with known metabolic sensitivities in early gestation.
While these mechanistic links remain hypothetical, the convergence of statistical association, gene function, and biological plausibility provides a coherent starting point for targeted experimental investigation. Importantly, the current data cannot exclude alternative explanations, including paternal genetic contributions, environmental exposures, or subtle population stratification effects, all of which warrant further study. Experimental follow-up—ranging from functional genomics in model organisms to in vitro studies of endometrial biology—will be critical to determine whether these loci exert direct causal effects or act in concert with other maternal and paternal factors.
Placing these results in an evolutionary context, the existence of heritable maternal influences on sex ratio aligns with longstanding hypotheses in evolutionary biology, such as the Trivers–Willard model, which predicts that parents in different physiological states may bias offspring sex to maximize reproductive fitness. The identification of specific maternal genetic variants now allows these theories to be tested in molecular terms. In doing so, this work reframes a century-old narrative of sex determination as a dynamic interplay between maternal and paternal biology—one shaped not by a single chromosomal event, but by a network of genetic and physiological processes operating before and during the earliest stages of life.
Genetic Testing for Boy or Girl Predisposition: Ethical Considerations
The recognition that maternal genetics can influence the sex ratio of offspring complicates a narrative that has been presented in biology classrooms for more than a century. As genetic tests for predicting whether you'll have all boys or all girls become available, society must grapple with how to use this information responsibly. Although the genetic effect detected here is subtle—altering probabilities rather than determining outcomes—it could, in the aggregate, shape population-level sex ratios over time. This is particularly relevant in societies where entrenched cultural or economic preferences for one sex already distort demographic balance; even a small biological bias could interact with these preferences, amplifying disparities across generations.
For genetic counseling, these findings introduce both opportunity and responsibility. In principle, knowledge of heritable sex-biasing variants could inform family planning, but the probabilistic nature of the effect demands careful communication to prevent overinterpretation or the misperception that these variants "decide" the sex of a child. Misunderstanding such results could reinforce gender-based expectations or inadvertently stigmatize families with unisexual sibships.
The ethical stakes extend beyond the clinic. As genomic screening becomes more widespread, the possibility arises that information about sex-biasing alleles could be misused for non-medical sex selection—an issue already fraught with cultural, political, and moral controversy. Policymakers, ethicists, and scientists will need to collaborate proactively to set clear boundaries and safeguards that protect against discrimination, respect reproductive autonomy, and ensure that genetic insights are not co-opted for purposes at odds with public health or equity.
Ultimately, the societal impact of this research will depend as much on how the science is communicated as on the underlying biology. Framing these results within their appropriate probabilistic context, resisting deterministic narratives, and embedding them in a broader conversation about equity and diversity will be essential to ensuring that this discovery becomes a catalyst for understanding rather than a source of division.
Frequently Asked Questions About Genetic Testing for Having All Boys or Girls
Can a genetic test predict if I'll have all boys or all girls?
Based on this Harvard research, genetic tests could potentially identify variants in genes like NSUN6, TSHZ1, and CYP2U1 that influence whether women are more likely to have children of one gender. While not yet commercially available, such tests are scientifically feasible.
What genes determine if you have boys or girls?
While the father's sperm (carrying X or Y chromosome) determines the baby's sex, this study shows maternal genes including NSUN6 (linked to having all girls), TSHZ1 (linked to having all boys), and CYP2U1 also influence the probability.
Is there a genetic reason some families have all boys or all girls?
Yes, this Harvard study provides the first genome-wide evidence that maternal genetic variants can bias offspring sex ratios, explaining why some families have children of predominantly one gender beyond random chance.
How accurate would a genetic test be for predicting baby gender patterns?
The study found that women with certain genetic variants had a 58-61% probability (compared to 50% baseline) of their next child being the same sex as previous children. While significant, this is a modest increase, not a guarantee.
When will genetic testing for sex ratio bias be available?
While the scientific foundation exists, commercial genetic tests for predicting whether you'll have all boys or girls are not yet available. Development would require additional validation studies and regulatory approval.
Conclusion
This study moves the question of human sex determination beyond a century-old narrative that placed fathers at the sole helm of biological destiny. By integrating vast reproductive histories with genome-wide analysis, the findings reveal that mothers, through their own genetic architecture, can subtly but measurably tilt the odds toward sons or daughters. The discovery of loci such as NSUN6, TSHZ1, and CYP2U1 is not the end of the story, but the beginning of a deeper inquiry into how maternal biology influences the earliest moments of life.
These results expand the framework of reproductive genetics, challenging long-standing assumptions and opening a research frontier that links molecular biology, evolutionary theory, and population health. They also prompt a necessary societal conversation: how will we navigate the knowledge that sex at birth is not purely random, and that maternal genes can play a quiet but persistent role? The answer will require a balance of curiosity and caution, innovation and restraint, as science continues to reveal the complex choreography between mother, father, and fate.

